
Depression is a complex disease with multiple contributing causes, including a variety of lifestyle, dietary, environmental, and genetic factors. While we still don’t fully understand what causes depression, scientists have recently discovered a variety of new potential links between gut health, inflammation, and the brain — and some early evidence suggests that these links may even play a significant role in behavior and mental health. Read on to learn more about what the latest science has to say about these potential connections, and the mechanisms that might be potentially involved!
What is Depression?
Depression — also referred to as Unipolar Depression and Major Depressive Disorder — is a complex disease with many contributing factors, including a wide variety of lifestyle, dietary, genetic, and environmental factors.
In short, we still don’t completely understand what causes depression. Moreover, conflicting results have arisen from studies that look at the effectiveness of antidepressants for the treatment of depression.
The only effective treatments — neurotransmitter reuptake inhibitors — still have only relatively low efficacy, as up to 30-40 % of patients do not respond to these drugs, and 60-70% of patients do not experience full remission [1, 2].
In addition, these medications can take a long time to become effective even in patients who do respond well to them; and many patients experience a number of severe side-effects while taking them.
Diagnostic Criteria for Depression
The primary diagnostic criteria for depression include [2]:
- Depressed or irritable mood
- Decreased interest in pleasurable activities, and/or the inability to experience pleasure
- Significant weight gain or loss (>5% change in a month)
- Insomnia or hypersomnia
- Psychomotor agitation or retardation
- Fatigue or loss of energy
- Feelings of worthlessness or excessive guilt
- Diminished ability to think or concentrate
- Recurrent thoughts of death or suicide
However, note that not every symptom is required to be present in order to be diagnosed with depression: most patients only have a subset of the full “core” symptoms.
Known Risk Factors for Depression
Some of the known factors that can increase a person’s risk of experiencing depression include [2]:
- The highest-risk age group is people between the ages of 25-30
- Females are approximately twice as likely to have depression than males
- People who are divorced, separated, or widowed have been reported to have relatively higher risks of depression compared to people who are married or who were never married
- People with annual incomes of less than $20,000/year may be at increased risk, and overall rates of depression tend to decrease as a person’s income increases (but only up to a point)
- Having relatives with early-onset major depression
- Early trauma or stressful life events
- Other (“comorbid”) health conditions — such as cardiovascular disease, AIDS, respiratory disorders, cancer, and Parkinson’s disease — have also been reported to increase a person’s overall risk of experiencing depression
Is Depression Really Caused by Low Serotonin?
Neurotransmitters are chemicals that our neurons use to transmit signals from one cell to another. There are over 100 different types of neurotransmitters, although some of the most well-known ones include serotonin, dopamine, and norepinephrine.
The majority of the most common or widely-used antidepressant medications (such as citalopram and many others) generally act by increasing the amount of the neurotransmitters serotonin and norepinephrine available in the brain (i.e. selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, or “SSRIs” and “SNRIs”, respectively).
However, some other antidepressants — such as bupropion (Wellbutrin) — act primarily on dopamine and norepinephrine (i.e. norepinephrine-dopamine reuptake inhibitors, or “NDRIs”).
Nonetheless, it remains an unanswered question whether low levels of serotonin or norepinephrine directly cause depression. For example, numerous scientific attempts to confirm that depressed people actually have abnormally low levels of these neurotransmitters have sometimes failed to fully verify a connection [3, 4].
Another major methodological limitation in our understanding of depression is that although antidepressants have established biochemical mechanisms (such as inhibiting monoamine oxidase (MAO), or inhibiting the reuptake of specific neurotransmitters), both the diagnosis and treatment of depression are based largely on subjective reports of symptoms, and not on any discrete measurements of specific biochemical markers or other “objective” biological indicators [2]. In other words, it’s not as simple as measuring a person’s level of these compounds, and then concluding whether they “have” depression or not.
Furthermore, while most SSRIs immediately increase serotonin levels and activity in the brain, many patients don’t report experiencing a significant improvement in their mood until being on their medication for several weeks or months [3]. Findings such as these suggest that serotonin levels may merely be the first link in a much more complex chain of mechanisms and biological changes that are collectively involved in depression — and serotonin may just be one piece in this larger puzzle.
The biological mechanisms involved in depression are still something of a mystery. Nonetheless, while the “serotonin deficiency” hypothesis of depression is still controversial and not 100% universally accepted by every researcher out there, at the present moment it is generally considered to be the best working hypothesis based on the overall evidence and data available so far.
Genetics of Depression
Just as the attempts to identify specific biochemical “markers” of depression have failed, so too have many of the attempts to identify specific genes or genetic mutations responsible for depression been met with only limited success — possibly because different cases of depression may be caused by mutations in many different genes, as well as unique environmental factors [5].
Several comprehensive studies (including genome-wide association studies, as well as familial and twin studies) have reported evidence suggesting that interactions between large numbers of individual genes — as well as the complex interactions of these genes with certain environmental factors — may be partly responsible for some cases or types of depression [6].
For example, one systematic genome-wide association study (GWAS) reported that a number of genes related to immune system function and inflammation may be among the possible genetic factors that influence a person’s risk of depression [7].
Another comprehensive GWAS study reportedly identified a number of genes related to serotonin function, circadian rhythm, and other neurotransmitters as possible genetic risk factors for depression, as listed in this table [8]:
| Gene Name | Variant | Minor Allele Frequency |
| 5HTTLPR/SLC6A4 | 44bp ins/del | 0.43 |
| intron 2 VNTR | 0.35 | |
| ACE | Ins/del intron 16 | 0.45 |
| BDNF | rs16917204 | 0.24 |
| rs2030324 | 0.46 | |
| rs988748 | 0.26 | |
| rs694 | 0.43 | |
| CLOCK | rs1801260 | 0.22 |
| COMT | rs4680 | 0.39 |
| DRD3 | rs6280 | 0.45 |
| DRD4 | 48 bp ins/del | 0.45 |
| GABRA3 | CA repeat intron 8 | 0.29 |
| GNB3 | rs5443 | 0.48 |
| HTR1A | rs6295 | 0.48 |
| HTR1B | rs6296 | 0.35 |
| HTR2A | rs6311 | 0.44 |
| rs6313 | 0.43 | |
| HTR2C | rs6318 | 0.17 |
| HTR6 | rs1805054 | 0.17 |
| MAOA | VNTR promoter | 0.34 |
| MTHFR | rs1801133 | 0.32 |
| NET/SLC6A2 | rs5569 | 0.27 |
| rs2242446 | 0.26 | |
| DAT/SLC6A3 | VNTR 3-UTR | 0.48 |
| TPH1 | rs1800532 | 0.36 |
| ACMSD [9] | rs2121337 |
Family studies of the recurrent/unipolar form of major depressive disorder (“MDD-RU”) have reported that first-degree relatives of people with depression diagnoses may be at particularly increased risk [10].
The serotonin transporter gene (SLC6A4) has also been associated with MDD [11]. SLC6A4 and other genes involved in the brain’s serotonergic system are now considered to be “candidate genes” for susceptibility to depression, which also fits in with the fact that many of the most common antidepressant medications are believed to act primarily on this system.
Five other “candidate genes” for depression risk include APOE (apolipoprotein E), DRD4 (dopamine receptor D4), GNB3 (guanine nucleotide binding protein subunit beta 3), MTHFR (methyltetrahydrofolate reductase), and SLC6A3 (sodium-dependent dopamine transporter) [12].
The Gut Microbiome
For every cell in a human body, there are ten cells of gut microorganism. The average human gut is inhabited by about 10,000-100,000 billion microorganisms including various species of bacteria, fungi, and even viruses. Altogether, the number of genes contained by the gut microbiome (i.e. all the genes of these various microorganisms) contains 150 times as many genes as the human genome itself [13].
Three major phyla (groups) of bacteria live in the large intestine: bacteroides, firmicutes, and actinobacteria. Most of these bacterial species don’t require oxygen to grow (i.e. they are “anaerobic” life forms).
Many different factors play a role in influencing exactly which species of microorganisms form our full gut microbiome. Some of these factors include [14]:
- Our mother’s gut bacteria
- Our diet
- Many medications
- Infections and other diseases
- Neurotransmitters
- Hormones
- Environment
- Stress
To make things even more complicated, our gut microbiome is constantly changing in response to many different environmental factors, even while our genetic factors remain unchanged. This is because bacteria have very short lifespans, and also compete for resources and interact among themselves.
Diet Affects the Gut Microbiome
Among the many different factors that can influence the composition of our gut microbiome, dietary factors are among the most widely studied by scientists.
A wide range of early scientific evidence suggests that various aspects of our diets can have a significant effect on exactly what bacterial species and populations form our gut microbiome.
For example, one animal study in mice has reported that certain dietary changes — such as including 50% lean beef compared to normal chow — significantly changes the makeup of the bacteria found in feces [15].
Additionally, one interesting study has reported that switching from a meat-heavy to a vegetarian diet can cause the gut microbiome to rapidly change from a “carnivorous” to an “herbivorous” profile within the span of just one day [16].
Interestingly, certain changes in the gut microbiome have been reported to change behavior: for example, in the mouse study above, the lean-beef diet was associated with a significant reduction in anxiety-like behaviors over the course of three months [15].
While there is still a lot we don’t understand about how the gut microbiome interacts with or influences the brain and behavior, the preliminary evidence reported so far suggests the potential for a significant connection that scientists are continuing to actively explore.
Neurotransmitters and Hormones vs the Gut Microbiome: A Two-Way Interaction
Some early evidence also suggests that the levels or activity of various neurotransmitters and hormones in the body and brain may also affect the gut microbiome in potentially-significant ways.
For example, one preliminary animal study reported that mice that experienced significant social stress (“social disruption”) showed reduced diversity in their gut microbiome compared to unstressed mice [17].
In addition, mice raised in a relatively germ-free environment have been reported to show elevated turnover rates of many neurotransmitters, as well as altered expression of several genes related to synaptic plasticity. Some researchers have taken these findings as suggesting that the makeup and activity of the gut microbiome may also modulate some aspects of brain development and behavior [18].
In short, the evidence so far suggests that humans and gut microorganisms have a complex, two-way relationship.
Additionally, some researchers have proposed that the interactions between the gut microbiome and the rest of the body or brain may even play a role in the development (etiology) of certain diseases and other health conditions.
Diseases potentially influenced by the gut microbiome:
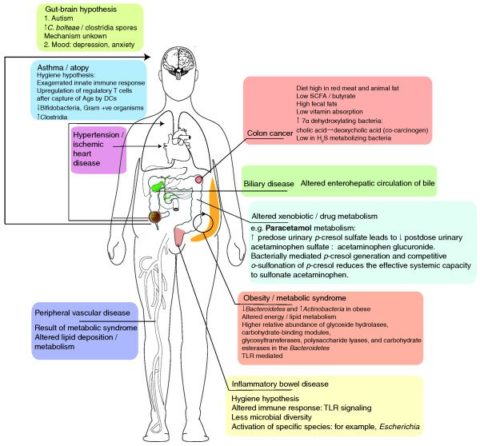
(Image source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3092099/)
The Gut Microbiome and Depression
The Gut-Brain Axis
The “gut-brain axis” refers to the hypothesized “two-way” connection between the gut’s microbiome and the brain [19].
The gut-brain axis may include any number of different potential mechanisms, including various neural, chemical, humoral and immunological signals that arise between the many different organ systems and individual bacterial species involved.
While many different diseases and health conditions could potentially be influenced by the gut-brain axis, some researchers have focused on the possible role of gut-brain interactions in the development of depression, in particular.
While the overall evidence so far is still in a very early stage, a number of preliminary findings may offer support for the possibility of a connection between the two.
Gut Bacteria May Influence Brain Development
According to one early animal study, rodents that have grown without gut bacteria (germ-free rats and mice) lack a mature enteroendocrine system (the hormone-production system of the gastrointestinal tract). They also differ in their level and activity of several different major neurotransmitters, compared to their counterparts with more typical levels of gut bacteria [20].
Manipulation of Gut Bacteria in Mice May Affect The Brain and Behavior
One preliminary animal study has reported that mice without gut bacteria may exhibit increased spontaneous movements, which the authors of this study interpreted as indicating greater anxiety. They further hypothesize that these behavioral differences may possibly be due to differences in the way that certain neurotransmitters — including serotonin, dopamine, and norepinephrine (noradrenaline) — are synthesized and metabolized throughout the brain [18].
Another study looked at the potential relationship between gut microorganisms and early-life stress. In this study, newborn rats who experienced stress and depression-like symptoms due to early-life maternal separation had several of these symptoms reduce when a particular species of bacteria (Bifidobacterium) was added to their diet. Although these reductions in depression-related behaviors were smaller than those caused by the conventional antidepressant medication citalopram, this preliminary finding nonetheless suggests that it may be theoretically possible to “treat” depression by changing the composition of the gut microbiome [21].
Some other early — but intriguing — evidence from animal studies suggests that even complex behaviors may be “copied” from one population of mice to another by “transplanting” feces (which often contains similar bacterial populations as those which are present in an animal’s gastrointestinal tract) [22].
Finally, another animal study has reported that antibiotics — which change the gut microbiome by killing off some of the bacterial species within it — may affect the amount of “exploratory behaviors” that mice exhibit, and may even change the levels of certain important compounds in the brain, such as brain-derived neurotrophic factor (BDNF) [22].
Probiotic Supplementation May Affect Depression Symptoms in Humans
You’ve probably heard of “probiotics” before — this refers to foods or dietary supplements that deliver and introduce specific strains (species) of bacteria into the gastrointestinal tract.
Some preliminary evidence suggests that using probiotics to “modify” the gut microbiome in humans may have some psychological effects.
For example, certain probiotic supplements have been reported to decrease depression symptom severity scores in both healthy non-elderly individuals, as well as non-elderly patients with diagnoses of major depressive disorder (MDD) [23].
Similarly, another early study has reported that the probiotics containing the bacterial strains L. helveticus and B. longum may have helped reduce depression in healthy volunteers when they were taken regularly [23].
Finally, one other preliminary study reported that a combination of the bacterial strains L. acidophilus, L. casei, and B. bifidum may help partially reduce depression symptoms [24].
While the potential mechanisms behind these effects are not yet known or understood, some researchers have noted that these “probiotic treatments” may result in lower insulin levels and insulin resistance, and hs-CRP, as well as increased levels of glutathione (a major natural anti-oxidant compound) in patients with major depressive disorder [24].
Overall, while this early research is promising and exciting, much more research will be needed to fully confirm these effects, as well as find out which mechanisms could potentially be responsible for these intriguing effects.
The Gut Microbiota-Brain Connection and Depression
Inflammation and Depression
The cytokine hypothesis of depression came about from the observation that several symptoms of depression resemble that of “sickness behaviors,” such as lethargy, fever, reduced appetite, decreased interest in exploratory behavior or sexual activity, and increased time spent sleeping [25].
Some researchers have proposed that evolutionarily speaking, sickness behaviors may make sense insofar as many of them may facilitate healing or reduce the transmission of disease. For example, the seclusion of a sick person may reduce the odds of them spreading an infection [25].
In connection with this, some research has reported that there are higher incidences of immune activation in patients with depression [25].
Relatedly, some other research has reported that patients treated with cytokine therapy (such as by using interferons and interleukin-2) often experience depression as a side-effect of this immune-stimulating treatment [26].
According to some reports, people who have health conditions involving gastrointestinal inflammation also have statistically increased rates of depression and anxiety symptoms [27]. Some researchers have estimated that up to 50-90% of IBS patients also have a co-occurring psychiatric disorder of some kind [28].
Although SSRIs (selective serotonin reuptake inhibitors) generally do not reduce “sickness behaviors” directly, they have been reported to reduce pro-inflammatory cytokines, and increase anti-inflammatory cytokines — mechanisms which would also be consistent with the cytokine hypothesis of depression [29, 30, 31].
Furthermore, patients with cytokine-induced depression often respond well to SSRIs [26].
However, there are several nuanced differences between the formal diagnostic criteria for depression (as defined by the DSM) and “sickness behavior” per se — and evidence is conflicting regarding whether inflammation really causes depression [26].
Additionally, not all ill patients have depression, and not all depression patients have high inflammatory markers. Altogether, this suggests that inflammation, possibly similar to serotonin deficiency, may only be one contributor to depression, rather than a sole direct cause [25]. In other words, abnormal regulation of inflammatory cytokines may simply be one piece of a much more complex puzzle — and much more research will be needed to fully explore the mechanisms involved in these potential connections.
Gastrointestinal Permeability (“Leaky Gut”) and Depression
The intestinal mucosal barrier and the mucosal immune system are two of the main mechanisms that help keep the gut microbes inside the gut lumen from interacting directly with the gut’s immune system. If these barriers are compromised, this may allow the bacteria to activate the immune system, which can in turn trigger inflammation [32].
(The condition in which these barriers are compromised is sometimes (unofficially) referred to as “leaky gut.”)
When bacteria move through the intestinal barrier, activation of the gut immune system can stimulate the production of pro-inflammatory cytokines. For example, one animal study has reported that mice with compromised gut barriers display increased anxiety-related behaviors, and that these behaviors subside once the barriers are restored, or when probiotics are introduced [33].
Although the mechanisms behind this phenomenon are not yet known for certain, some researchers have suggested that “bad” gut bacteria may trigger the inflammation through the TLR4 receptor [34].
According to the authors of one preliminary study in humans, serum antibodies such as IgA and IgM typically get activated to fight against harmful gut bacteria. The presence of these antibodies, therefore, is one potential biological indicator of compromised gut barriers — and one study even reported that elevated levels of these antibodies may be used to identify depression patients with up to 90% accuracy [35].
Nonetheless, these mechanisms and their effects are extraordinarily complex, and much more research will be needed to verify these preliminary findings.
Gut Bacteria and the Stress Response
According to some early reports, depressed patients have statistically higher rates of dysfunctions of the stress response system (such as the HPA axis). Additionally, resolution of the HPA axis dysfunction correlates well with remission (“cure”) of depression, suggesting some mutual link between them [36].
Monoamine reuptake inhibitors (MAO-Is) have been reported to decrease glucocorticoid receptor resistance, thus reducing HPA axis dysfunction. This has led some researchers to suggest that drugs that target the brain’s HPA axis may be potentially-effective treatments for major depression [36].
Additionally, early life stress has been associated with an increased risk for depression — at least partly because early life stress may make the person more sensitive to stress later on [37].
Colonization of the gut flora may influence how sensitive an organism is to stress in general. For example, one early animal study has reported that adult mice without gut bacteria who were exposed to mild restraint stress exhibited a significantly stronger stress response compared to mice with more typical gut bacteria microbiomes [38]. Furthermore, this amplified stress response in the mice without gut bacteria was reportedly reduced by giving these mice a probiotic bacteria strain called Bifidobacterium infantis [38].
Similarly, one animal study in rats reported that stress from taking newborns away from the mother (maternal separation stress) may lead to dramatic long-term changes in the offspring’s intestinal microbiome [39, 40, 41].
Although the mechanisms behind these effects are still mysterious, some researchers have proposed that the introduction of certain probiotic strains may counteract the stress response by reducing intestinal permeability, and suppressing excessive inflammatory responses [40, 42].
All in all, while these early findings are promising, much more research will still be needed to figure out for sure if these mechanisms are relevant to human health, and exactly how much of an influence they might ultimately have.
How Gut Bacteria May Affect The Brain
Gut Bacteria May Modulate Neurotransmitter Production
The gut bacteria are major modulators of chemicals (metabolites) in the blood. Some of these are “ingredients” or “building blocks” (metabolic precursors) for making neurotransmitters in the brain.
In other words, this means that gut bacteria may be able to have some influence on the levels and activity of certain neurotransmitters — which may, in turn, give them the ability to influence brain activity and behavior (albeit indirectly).
For example, carbohydrate fermentation by gut microbes results in the creation of short-chain fatty acids, such as propionates and butyrates. These metabolites may have neuroactive properties, and some research has even implicated them in the development or symptoms of autism spectrum disorders (ASDs) [43].
Relatedly, some early behavioral experiments in mice suggest that sodium butyrate — another short-chain fatty acid — may mimic some of the effects of classical antidepressant drugs. However, these preliminary findings have been somewhat conflicting, with other studies reporting no similarities in their effects [44, 45].
Gut Bacteria, Inflammation, and Serotonin
The amino acid tryptophan is one of the major “building blocks” (metabolic precursors) that the brain uses to produce the neurotransmitter serotonin. While tryptophan depletion does not always lead to depression, some researchers have suggested that low serum tryptophan may sometimes precipitate depression in susceptible individuals [26].
Some research indicates that increases in the level of certain pro-inflammatory cytokines — such as IFN-α, IFN-γ, and TNF-α — may stimulate the activity of the enzyme indoleamine-2,3-dioxygenase (IDO), which in turn stimulates the conversion of tryptophan into neurotoxic compounds, including kynurenine and quinolinic acid [46].
Based on this, some researchers believe that it may be these neurotoxic substances — rather than tryptophan depletion per se — that may contribute to the development of depression [46].
Gut bacteria and inflammation may affect serotonin metabolism:
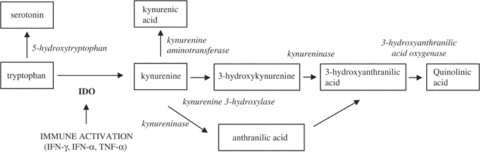 [/caption]
[/caption]
Kynurenine of the tryptophan metabolic pathway from [47].
(Image source: https://www.ncbi.nlm.nih.gov/pubmed/15494706/)
According to one animal study, rats treated with Bifidobacterium infantis are reported to have reduced inflammatory cytokines, increased levels of tryptophan and kynurenic acid (a neuroprotective metabolite of tryptophan), and reduced kynurenine levels in comparison to untreated rats [48].
In addition, these effects of B. infantis on tryptophan metabolism may be strain-specific, as similar effects were not observed in response to treatment with other bacterial strains, such as B. longum. However, B. longum has been reported to reduce depression (and increase BDNF levels) according to at least one other study in rats [49].
While this study offers promising evidence that gut bacteria may help modulate tryptophan metabolites (and hence potentially prevent depression), more research will be required to verify this mechanism’s possible role in depression.
Gut Bacteria, Enterochromaffin Cells, and the Vagus Nerve
Finally, one of the other mechanisms that the gut microbiome may use to communicate with the central nervous system is through enterochromaffin cells (ECCs) and the vagus nerve.
For example, some researchers currently believe that enterochromaffin cells and the vagus nerve may be involved in the communication between the gut bacteria and the brain, although we still don’t completely understand how [19].
Enterochromaffin Cells (ECC) Sense Gut Bacteria and Secrete Serotonin:
- ECCs are found throughout the digestive tract
- They may play a role in detecting the various types of bacteria and foods in the gut (via “toll-like receptors”)
- In turn, these cells may secrete serotonin and other signaling peptides in response to various stimuli (such as certain foods, microbial factors, or bacterial toxins)
- Serotonin secreted by ECC cells stimulates gastrointestinal movement; so pathogenic bacteria tend to increase serotonin signaling in the gut to induce a “flushing” motion, which may induce diarrhea or vomiting.
- ECCs also harbor receptors for the corticotropin-releasing hormone (CRH), as well as various major neurotransmitters such as GABA, acetylcholine, and epinephrine (adrenaline)
The Vagus Nerve May Link Between the Gut and the Brain:
- The vagus nerve is believed to play a role in controlling the movement of food through the intestinal tract (peristalsis), as well as stimulating the secretion of various compounds used to communicate between gut cells and the gut’s native microorganisms.
- The vagus nerve may also play a role in the secretion of serotonin by ECCs (see above).
- This nerve connects to (“innervates”) many regions of the brain, including the raphe nuclei, one of the main regions involved in the production and secretion of serotonin throughout the rest of the brain.
- Although this is not always the case, cutting the vagus nerve often diminishes the effects of probiotics on depression and anxiety in mice, such as in these two studies:
- Introducing Lactobacillus rhamnosus (JB1) altered gamma-aminobutyric acid (GABA) receptor function in the brains of mice, resulting in mice with higher anxiety and reduced depression [50].
- Mice with infectious colitis also display an anxiety-like phenotype, which can reportedly be normalized by introducing the bacterial strain Bifidobacterium longum (NCC 3301) into their diets. B. longum has been reported to reduce anxiety-like behaviors, although it was not reported to have any direct effects on the underlying colitis per se [51].
- The FDA has recognized vagus nerve stimulation (VNS) as a potential treatment for treatment-resistant depression in 2001. According to a small clinical trial in treatment-resistant patients with severe depression, the long-term response rates to this treatment was around 44%, with a remission rate of about 29% after one year of treatment [52].
- Unfortunately, scientists still don’t fully understand exactly how vagus nerve stimulation helps with depression, or what the underlying mechanisms are. Nonetheless, the successful treatment outcomes of vagus nerve stimulation observed in this preliminary clinical trial suggest that the vagus nerve may act as a key modulator in the communication between the gut and the brain, although other organs controlled by the vagus nerve could also contribute to this pathway.
- Another study which tracked HPA axis dysfunction in depressed patients reported that HPAA dysfunctions were also resolved by VNS, along with the depression symptoms [53].
